Abstract
Introduction The successive International Guidelines (Borowitz. et al. Cytometry B Clin Cytom 2010; Illingworth. et al. Cytometry B Clin Cytom 2018) recommend both detection and follow-up of rare PNH cells using high-resolution flow cytometry (FCM) analysis. These rare PNH cells, defined as GPI-deficient cells ranging from 0.01% to <0.1%, are distinguished from minor PNH clone, ranging from 0.1% to 1%. However, clinical relevance as well as long-term evolution of such rare PNH cells are still unknown.
Objective We aimed to compare clinical and biological presentation of patients with rare PNH cells or a minor PNH clone along with long-term follow-up using data provided by the 5-year French nation-wide multicenter observational study, the Observatory of PNH Clones.
Methods All patients of any age with a newly or previously diagnosed PNH clone or GPI-deficient cells ≥0.01%, detected in France by FCM from February 29th, 2016 to February 28th, 2021 were eligible for inclusion in this Observatory, provided that the center had participated in the inter-laboratory comparison program ongoing since 2013 (Debliquis et. al. Am J Clin Pathol 2015). For each patient, the baseline assessment was always considered as the initial PNH clone detection, even if it occurred before the initiation of the Observatory. Thus, this strategy allowed the collection of cases with long-term follow-up. A referent cytometrist at each center included patients in the e-CRF available on the CytHem-HPN website providing clinical and biological information as well as FCM raw data files. This study was approved by the national research ethics board.
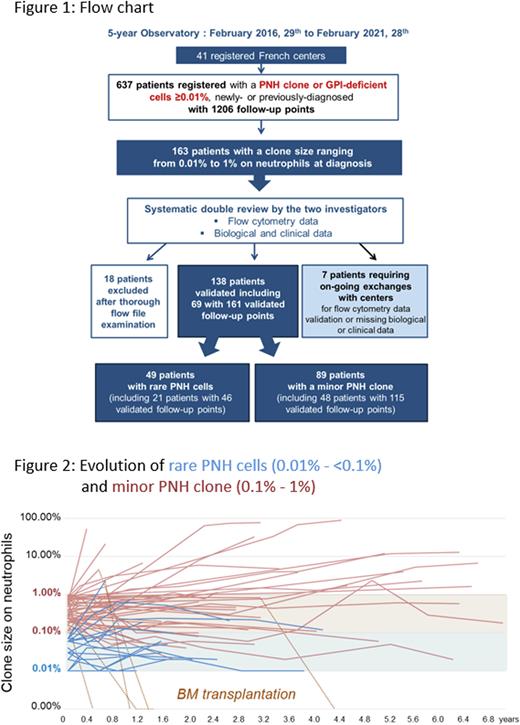
Results As of February 28th, 2021, 41 registered flow cytometry laboratories across France enrolled 637 patients with a PNH clone or GPI-deficient cells ≥ 0.01%. Selection of patients displaying rare PNH cells (i.e., o.o1% to <0.1%) or a minor PNH clone (i.e., 0.1% to 1%) on neutrophils led to the re-examination of 163 patient FCM files (provided by 26 centers) by the two principal investigators. FCM data and the e-CRF filling were both thoroughly reviewed leading to the update of roughly half of the submitted files. This enabled the validation of 138 patients at diagnosis and the exclusion of 18 (11%) patients who did not display a genuine PNH clone according to the international recommendations (at least 20 clustered events). For 7 of 163 selected patients, exchanges with centers are still ongoing for FCM data validation or due to missing clinical or biological data. Sixty-nine of the 138 validated patients displayed at least one follow-up point (Figure 1). Thus, we were able to compare 49 patients with rare PNH cells and 89 patients with a minor PNH clone with a significantly different clone size on the 3 lineages (median size on neutrophils: 0.03 [0.01 - 0.09] vs 0.34 [0.1 - 0.97] ; p<0.0001, on monocytes: 0.03 [0 - 7.88 ] vs 0.50 [0 - 7.08] ; p<0.0001, on red blood cells: 0.01 [0 - 1.36 ] vs 0.08 [0 - 1.24]; p= 0.0096). The patient median age at diagnosis was similar for both groups, 59 years [6 - 91] with 4 pediatric cases (<18y) for "rare PNH cells” group vs 49 years [10 - 87] with 9 pediatric cases for "minor PNH clone” group and a M/F sex ratio of 1.45 vs 0.98. Diagnoses were made between 2010 and 2021 with clinical information available in 98% of cases (n=47 and 88). Ninety-two to 94% of patients from both groups displayed BM failure including mainly aplastic anemia (n=30/47 vs 61/88), myelodysplastic syndrome (n=2 vs 6) and unexplained cytopenia(s) (n=11 vs 16). Only 4 cases of thrombosis for "rare PNH cells” group vs 3 for "minor PNH clone” group were observed, and the 2 cases of hemolytic anemia belong to the latter group. A more severe thrombopenia was observed in the "minor PNH clone” (68 [2-356] vs 44 [2-308] G/l) while all other biological parameters showed no significant difference. Long-term follow-up up to 7.0 years showed that one third of patients from both groups displayed an increase in clone size (above 0.1% for "rare PNH cells” and above 1% for "minor PNH clone", see Figure 2), most remained stable and a few decreased, the disappearance being always due to BM transplantation.
Conclusion This multicenter study based on robust FCM analysis showed no differences between the clinical and biological presentation of patients with either rare PNH cells or a minor PNH clone. Long-term follow-up showed that rare PNH cells can evolve into minor PNH clone, suggesting that these cases may be considered as genuine minor clones.
Disclosures
Le Garff-Tavernier:Abbvie: Consultancy, Honoraria; Janssen: Honoraria. Debliquis:Abbvie: Honoraria; Alexion: Consultancy, Honoraria; Sanofi: Honoraria; Takeda: Honoraria. Wagner-Ballon:Alexion: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal